This product is used for in vitro qualitative detection of the antigen of SARS-CoV-2 in human saliva specimen.
CE Certificated and Germany Regestied.
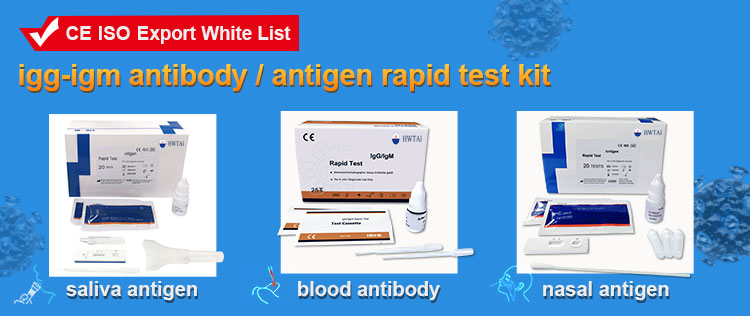

Introduction
1. Intend Use
This product is used for in vitro qualitative detection of the antigen of SARS-CoV-2 in human saliva specimen. The novel coronaviruses belong to the β genus. COVID-19 is an acute respiratory Infectious disease. People are generally susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection, asymptomatic infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main Manifestations include fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat, myalgia and diarrhea are found in a few cases.
2. Test Procedure
This kit uses the double antibody-sandwich method to detect SARS-CoV-2 antigens. When an appropriate amount of specimen is added to the specimen well(s) of the test device, the specimen will move forward along the test device. If the specimen contains an antigen, the antigen binds to mouse anti- SARS-CoV-2 N protein monoclonal antibody labeled with colloidal gold on the binding pad, and the immune complex forms a sandwich complex with another coated mouse anti- SARS-CoV-2 N protein monoclonal antibody which was coated on the test line, a visible colored line will show up, which indicates that the SARS-CoV-2 antigen is positive. The test device also contains a quality control line, regardless of whether there is a test line, the red quality control line should appear. If the quality control line does not appear, it indicates that the test result is invalid and need to do the test again.
3. Components



Contact: Neo
Phone: 008615867460640
E-mail: Info@Hwtai.com
Whatsapp:008615867460640
Add: Building 2, Xinmao Qilu Science Technology Industrial Park, Tianqiao District, Jinan City, Shandong Province,China.
We chat
